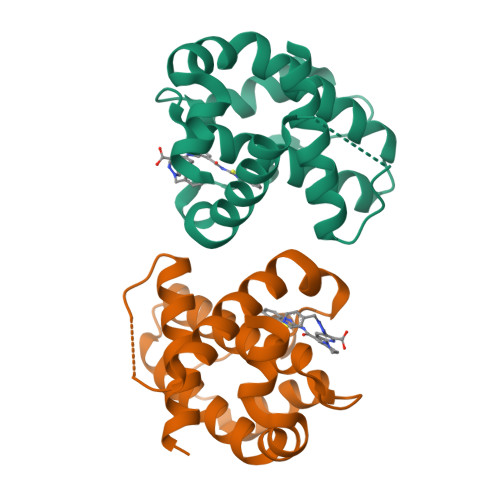
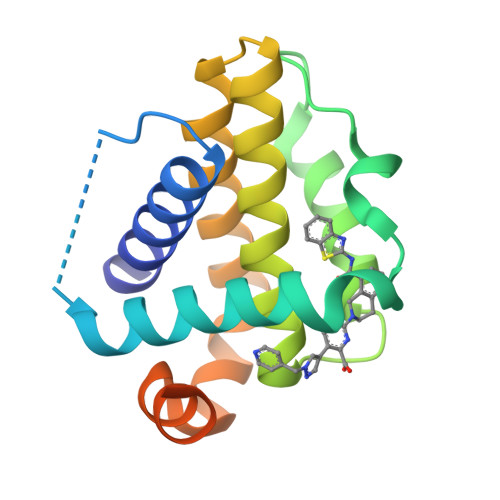
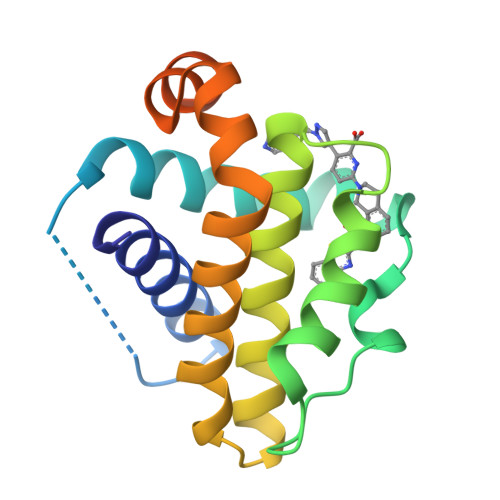
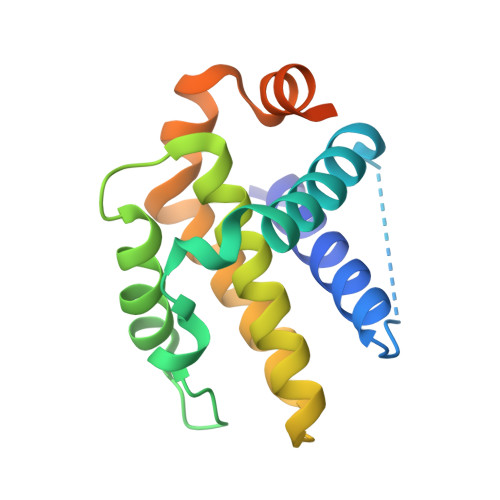
Discovery of A-1331852, a First-in-Class, Potent, and Orally-Bioavailable BCL-X L Inhibitor.
Wang, L., Doherty, G.A., Judd, A.S., Tao, Z.F., Hansen, T.M., Frey, R.R., Song, X., Bruncko, M., Kunzer, A.R., Wang, X., Wendt, M.D., Flygare, J.A., Catron, N.D., Judge, R.A., Park, C.H., Shekhar, S., Phillips, D.C., Nimmer, P., Smith, M.L., Tahir, S.K., Xiao, Y., Xue, J., Zhang, H., Le, P.N., Mitten, M.J., Boghaert, E.R., Gao, W., Kovar, P., Choo, E.F., Diaz, D., Fairbrother, W.J., Elmore, S.W., Sampath, D., Leverson, J.D., Souers, A.J.(2020) ACS Med Chem Lett 11: 1829-1836
- PubMed: 33062160
- DOI: https://doi.org/10.1021/acsmedchemlett.9b00568
- Primary Citation of Related Structures:
6VWC - PubMed Abstract:
Herein we describe the discovery of A-1331852, a first-in-class orally active BCL-X L inhibitor that selectively and potently induces apoptosis in BCL-X L -dependent tumor cells. This molecule was generated by re-engineering our previously reported BCL-X L inhibitor A-1155463 using structure-based drug design. Key design elements included rigidification of the A-1155463 pharmacophore and introduction of sp 3 -rich moieties capable of generating highly productive interactions within the key P4 pocket of BCL-X L . A-1331852 has since been used as a critical tool molecule for further exploring BCL-2 family protein biology, while also representing an attractive entry into a drug discovery program.
Organizational Affiliation:
AbbVie Inc., 1 North Waukegan Road, North Chicago, Illinois 60064, United States.